

- #Chemlab hydrate formula how to
- #Chemlab hydrate formula full
It loses two water molecules upon heating at 63 ☌ (145 ☏), followed by two more at 109 ☌ (228 ☏) and the … See more As a fungicide and herbicide Copper sulfate has been used for control of algae in lakes and related fresh waters subject to eutrophication.
Vitriol See more Copper(II) sulfate pentahydrate decomposes before melting. In nature, it is found as the very rare mineral known as … See more Copper sulfate can … See more Anhydrous copper(II) sulfate can be produced by dehydration of the commonly available pentahydrate copper sulfate. For laboratory use, copper sulfate is usually purchased. Older names for the … See more Copper sulfate is produced industrially by treating copper metal with hot concentrated sulfuric acid or copper oxides with dilute sulfuric acid. The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate. Ĭopper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula CuSO4. Your goal now is to calculate the % (by mass) of water in hydrated CuSO4, based on its correct formula (which is CuSO4 5 H2O ). Name your compound by filling in the appropriate prefix: “copper(II) sulfate _hydrate” 8. The way we name a hydrated ionic compound is by using the same prefixes as used for binary covalent compounds. How is the formula for hydrated copper(II) sulfate determined?Ĭopper Sulfate - Structure, Properties, and Uses of CuSO4 … WebFinding the formula of hydrated copper - The mass of water is found by weighing before and after - Studocu Lecture Notes with Q&A finding the formula of hydrated copper(ii) sulfate aim in this experiment, known mass of hydrated copper(ii) sulfate is heated to remove Skip to document Ask an Expert Sign inRegister Sign inRegister Home number to array labview This form is characterized by its … comercializadora ateca s.a. WebThe most common form of copper sulfate is its pentahydrate, given by the chemical formula CuSO 4. Hydrated copper sulphate formula mean (II)_sulfate It is useful to know the percent of water contained within a hydrate. The formula for water is set apart at the end of the formula with a dot, followed by a coefficient that represents the number of water molecules per formula unit. The name of the compound is cobalt (II) chloride hexahydrate and its formula is \(\ce\). Percentage of water of crystallisation in a compound Therefore, the most common hydrated form of it is CuSO 4. Moreover, this compound is generally found as a hydrated salt with between 1 to 5 molecules of water. 
Similarly, its molar mass is 159.60 g mol -1. WebThe chemical formula for Copper Sulfate is CuSO 4.
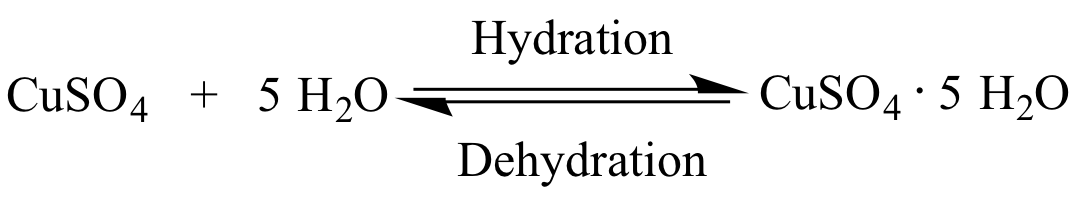
The difference in the mass of the hydrate and the mass of the anhydrous salt can be used to determine the formula of the compound.Hydrated copper sulphate formula number to array js The mass of the resulting anhydrous salt is determined. The premise of the lab is simple: A known mass of a hydrate is heated to release the water of hydration. So this past week we did a lab called " Composition of Hydrates." Hydrates are compounds that have some number of water molecules attached to them.
Lately, I have been teaching how to write chemical formulas and the naming of compounds. and I am being reminded how much I love them! I love the mathematical and analytical nature of chemistry labs and the need for exact and precise laboratory procedures.

So this year I am doing chemistry labs that I have not done for some time. My school made the transition this year from a 5 period day to a 7 period day, thus the need to assign teachers additional classes to teach.
My usual teaching assignment is a full day of AP Biology. This is the first time in about 7 years that I have taught a chemistry class.








 0 kommentar(er)
0 kommentar(er)
